DISEASE BURDEN
Metastatic Merkel cell carcinoma (mMCC) is an aggressive disease with poor survival after distant metastasis1,2
5-year survival by stage3
Stage I
62.8%
Stage II
38.4%to54.6%
Stage III
26.8%to40.3%
Stage IV
13.5%
MCC is rare, but the incidence is growing4,5
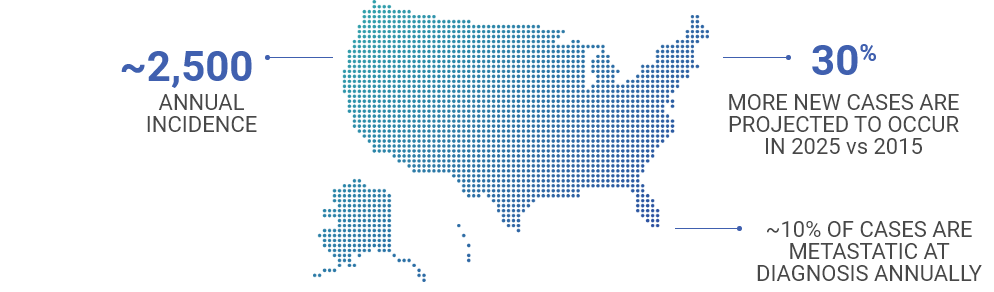
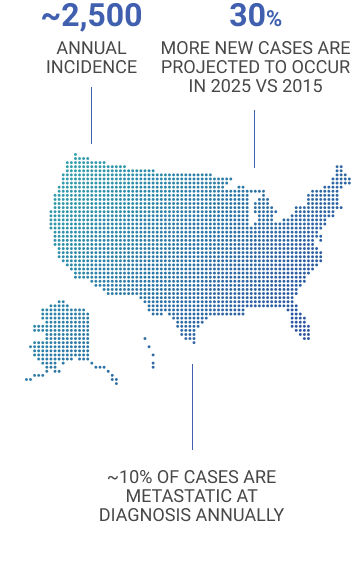
- Patients with early stage disease often experience recurrence and develop metastasis6,7
DIAGNOSING MCC
A
Asymptomatic
E
Expanding rapidly
I
Immunosuppressed
O
>50 years of age
U
UV-exposed
References: 1. Chapter 30, Merkel cell carcinoma. In: Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010:315-323. 2. Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63(5):751-761. 3. Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017 Oct 26;3:17077. doi:10.1038/nrdp.2017.77. 4. Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78:457-463. 5. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374-1385. 6. Poulsen M, Rischin D, Walpole E, et at; Trans-Tasman Radiation Oncology Group. High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study-TROG 96:07. J Clin Oncol. 2003;21(23):4371-4376. 7. Cowey CL, Mahnke L, Espirito J, et al. Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol. 2017;13(19):1699-1710.
IMPORTANT SAFETY INFORMATION AND INDICATIONS
IMPORTANT SAFETY INFORMATION
BAVENCIO can cause severe and fatal immune-mediated adverse reactions in any organ system or tissue and at any time after starting treatment with a PD-1/PD-L1 blocking antibody, including after discontinuation of treatment.
Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of PD-1/PD-L1 blocking antibodies. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
No dose reduction for BAVENCIO is recommended. For immune-mediated adverse reactions, withhold or permanently discontinue BAVENCIO depending on severity. In general, withhold BAVENCIO for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue BAVENCIO for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating corticosteroids. In general, if BAVENCIO requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy. Toxicity management guidelines for adverse reactions that do not necessarily require systemic corticosteroids (eg, endocrinopathies and dermatologic reactions) are discussed in subsequent sections.
BAVENCIO can cause immune-mediated pneumonitis. Withhold BAVENCIO for Grade 2, and permanently discontinue for Grade 3 or Grade 4 pneumonitis. Immune-mediated pneumonitis occurred in 1.1% (21/1854) of patients, including fatal (0.1%), Grade 4 (0.1%), Grade 3 (0.3%), and Grade 2 (0.6%) adverse reactions. Systemic corticosteroids were required in all (21/21) patients with pneumonitis.
BAVENCIO can cause immune-mediated colitis. The primary component of immune-mediated colitis consisted of diarrhea. Cytomegalovirus infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies. Withhold BAVENCIO for Grade 2 or Grade 3, and permanently discontinue for Grade 4 colitis. Immune-mediated colitis occurred in 1.5% (27/1854) of patients, including Grade 3 (0.4%) and Grade 2 (0.8%) adverse reactions. Systemic corticosteroids were required in all (27/27) patients with colitis.
BAVENCIO can cause hepatotoxicity and immune-mediated hepatitis. Withhold or permanently discontinue BAVENCIO based on tumor involvement of the liver and severity of aspartate aminotransferase (AST), alanine aminotransferase (ALT), or total bilirubin elevation. Immune-mediated hepatitis occurred with BAVENCIO as a single agent in 1.1% (20/1854) of patients, including fatal (0.1%), Grade 3 (0.8%), and Grade 2 (0.2%) adverse reactions. Systemic corticosteroids were required in all (20/20) patients with hepatitis.
BAVENCIO can cause primary or secondary immune-mediated adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement, as clinically indicated. Withhold BAVENCIO for Grade 3 or Grade 4 endocrinopathies until clinically stable or permanently discontinue depending on severity. Immune-mediated adrenal insufficiency occurred in 0.6% (11/1854) of patients, including Grade 3 (0.1%) and Grade 2 (0.4%) adverse reactions. Systemic corticosteroids were required in all (11/11) patients with adrenal insufficiency.
BAVENCIO can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism. Initiate hormone replacement, as clinically indicated. Withhold BAVENCIO for Grade 3 or Grade 4 endocrinopathies until clinically stable or permanently discontinue depending on severity. Immune-mediated pituitary disorders occurred in 0.1% (1/1854) of patients, which was a Grade 2 (0.1%) adverse reaction.
BAVENCIO can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement for hypothyroidism or institute medical management of hyperthyroidism, as clinically indicated. Withhold BAVENCIO for Grade 3 or Grade 4 endocrinopathies until clinically stable or permanently discontinue depending on severity. Thyroiditis occurred in 0.2% (4/1854) of patients, including Grade 2 (0.1%) adverse reactions. Hyperthyroidism occurred in 0.4% (8/1854) of patients, including Grade 2 (0.3%) adverse reactions. Systemic corticosteroids were required in 25% (2/8) of patients with hyperthyroidism. Hypothyroidism occurred in 5% (97/1854) of patients, including Grade 3 (0.2%) and Grade 2 (3.6%) adverse reactions. Systemic corticosteroids were required in 6% (6/97) of patients with hypothyroidism.
BAVENCIO can cause immune-mediated type I diabetes mellitus, which can present with diabetic ketoacidosis. Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Withhold BAVENCIO for Grade 3 or Grade 4 endocrinopathies until clinically stable or permanently discontinue depending on severity. Immune-mediated type I diabetes mellitus occurred in 0.2% (3/1854) of patients, including Grade 3 (0.2%) adverse reactions.
BAVENCIO can cause immune-mediated nephritis with renal dysfunction. Withhold BAVENCIO for Grade 2 or Grade 3, and permanently discontinue for Grade 4 increased blood creatinine. Immune-mediated nephritis with renal dysfunction occurred in 0.1% (2/1854) of patients, including Grade 3 (0.1%) and Grade 2 (0.1%) adverse reactions. Systemic corticosteroids were required in all (2/2) patients with nephritis with renal dysfunction.
BAVENCIO can cause immune-mediated dermatologic adverse reactions, including rash or dermatitis. Exfoliative dermatitis including Stevens Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/PD-L1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes. Withhold BAVENCIO for suspected and permanently discontinue for confirmed SJS, TEN, or DRESS. Immune-mediated dermatologic adverse reactions occurred in 6% (108/1854) of patients, including Grade 3 (0.1%) and Grade 2 (1.9%) adverse reactions. Systemic corticosteroids were required in 25% (27/108) of patients with dermatologic adverse reactions.
BAVENCIO can result in other immune-mediated adverse reactions. Other clinically significant immune-mediated adverse reactions occurred at an incidence of <1% in patients who received BAVENCIO or were reported with the use of other PD-1/PD-L1 blocking antibodies. For myocarditis, permanently discontinue BAVENCIO for Grade 2, Grade 3, or Grade 4. For neurological toxicities, withhold BAVENCIO for Grade 2 and permanently discontinue for Grade 3 or Grade 4.
BAVENCIO can cause severe or life-threatening infusion-related reactions. Premedicate patients with an antihistamine and acetaminophen prior to the first 4 infusions and for subsequent infusions based upon clinical judgment and presence/severity of prior infusion reactions. Monitor patients for signs and symptoms of infusion-related reactions, including pyrexia, chills, flushing, hypotension, dyspnea, wheezing, back pain, abdominal pain, and urticaria. Interrupt or slow the rate of infusion for Grade 1 or Grade 2 infusion-related reactions. Permanently discontinue BAVENCIO for Grade 3 or Grade 4 infusion-related reactions. Infusion-related reactions occurred in 26% of patients, including three (0.2%) Grade 4 and ten (0.5%) Grade 3 infusion-related reactions. Eleven (85%) of the 13 patients with Grade ≥3 reactions were treated with intravenous corticosteroids.
Fatal and other serious complications of allogeneic hematopoietic stem cell transplantation (HSCT) can occur in patients who receive HSCT before or after being treated with a PD-1/PD-L1 blocking antibody. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/PD-L1 blocking antibody prior to or after an allogeneic HSCT.
BAVENCIO can cause fetal harm when administered to a pregnant woman. Advise patients of the potential risk to a fetus including the risk of fetal death. Advise females of childbearing potential to use effective contraception during treatment with BAVENCIO and for at least 1 month after the last dose of BAVENCIO. It is not known whether BAVENCIO is excreted in human milk. Advise a lactating woman not to breastfeed during treatment and for at least 1 month after the last dose of BAVENCIO due to the potential for serious adverse reactions in breastfed infants.
The most common adverse reactions (all grades, ≥20%) in patients with metastatic Merkel cell carcinoma(MCC) were fatigue (47%), musculoskeletal pain (29%), infusion-related reaction (26%), rash (25%), nausea (23%), constipation (22%), cough (22%), and diarrhea (21%).
Laboratory abnormalities worsening from baseline (all grades, ≥20%) in patients with metastatic MCC were decreased lymphocyte count (51%), decreased hemoglobin (40%), increased aspartate aminotransferase (31%), decreased platelet count (23%), increased alanine aminotransferase (22%), and increased lipase (21%).
IMPORTANT SAFETY INFORMATION AND INDICATIONS
IMPORTANT SAFETY INFORMATION
BAVENCIO can cause severe and fatal immune-mediated adverse reactions in any organ system or tissue and at any time after starting treatment with a PD-1/PD-L1 blocking antibody, including after discontinuation of treatment.
Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of PD-1/PD-L1 blocking antibodies. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
No dose reduction for BAVENCIO is recommended. For immune-mediated adverse reactions, withhold or permanently discontinue BAVENCIO depending on severity. In general, withhold BAVENCIO for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue BAVENCIO for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating corticosteroids. In general, if BAVENCIO requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy. Toxicity management guidelines for adverse reactions that do not necessarily require systemic corticosteroids (eg, endocrinopathies and dermatologic reactions) are discussed in subsequent sections.
BAVENCIO can cause immune-mediated pneumonitis. Withhold BAVENCIO for Grade 2, and permanently discontinue for Grade 3 or Grade 4 pneumonitis. Immune-mediated pneumonitis occurred in 1.1% (21/1854) of patients, including fatal (0.1%), Grade 4 (0.1%), Grade 3 (0.3%), and Grade 2 (0.6%) adverse reactions. Systemic corticosteroids were required in all (21/21) patients with pneumonitis.
BAVENCIO can cause immune-mediated colitis. The primary component of immune-mediated colitis consisted of diarrhea. Cytomegalovirus infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies. Withhold BAVENCIO for Grade 2 or Grade 3, and permanently discontinue for Grade 4 colitis. Immune-mediated colitis occurred in 1.5% (27/1854) of patients, including Grade 3 (0.4%) and Grade 2 (0.8%) adverse reactions. Systemic corticosteroids were required in all (27/27) patients with colitis.
BAVENCIO can cause hepatotoxicity and immune-mediated hepatitis. Withhold or permanently discontinue BAVENCIO based on tumor involvement of the liver and severity of aspartate aminotransferase (AST), alanine aminotransferase (ALT), or total bilirubin elevation. Immune-mediated hepatitis occurred with BAVENCIO as a single agent in 1.1% (20/1854) of patients, including fatal (0.1%), Grade 3 (0.8%), and Grade 2 (0.2%) adverse reactions. Systemic corticosteroids were required in all (20/20) patients with hepatitis.
BAVENCIO can cause primary or secondary immune-mediated adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement, as clinically indicated. Withhold BAVENCIO for Grade 3 or Grade 4 endocrinopathies until clinically stable or permanently discontinue depending on severity. Immune-mediated adrenal insufficiency occurred in 0.6% (11/1854) of patients, including Grade 3 (0.1%) and Grade 2 (0.4%) adverse reactions. Systemic corticosteroids were required in all (11/11) patients with adrenal insufficiency.
BAVENCIO can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism. Initiate hormone replacement, as clinically indicated. Withhold BAVENCIO for Grade 3 or Grade 4 endocrinopathies until clinically stable or permanently discontinue depending on severity. Immune-mediated pituitary disorders occurred in 0.1% (1/1854) of patients, which was a Grade 2 (0.1%) adverse reaction.
BAVENCIO can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement for hypothyroidism or institute medical management of hyperthyroidism, as clinically indicated. Withhold BAVENCIO for Grade 3 or Grade 4 endocrinopathies until clinically stable or permanently discontinue depending on severity. Thyroiditis occurred in 0.2% (4/1854) of patients, including Grade 2 (0.1%) adverse reactions. Hyperthyroidism occurred in 0.4% (8/1854) of patients, including Grade 2 (0.3%) adverse reactions. Systemic corticosteroids were required in 25% (2/8) of patients with hyperthyroidism. Hypothyroidism occurred in 5% (97/1854) of patients, including Grade 3 (0.2%) and Grade 2 (3.6%) adverse reactions. Systemic corticosteroids were required in 6% (6/97) of patients with hypothyroidism.
BAVENCIO can cause immune-mediated type I diabetes mellitus, which can present with diabetic ketoacidosis. Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Withhold BAVENCIO for Grade 3 or Grade 4 endocrinopathies until clinically stable or permanently discontinue depending on severity. Immune-mediated type I diabetes mellitus occurred in 0.2% (3/1854) of patients, including Grade 3 (0.2%) adverse reactions.
BAVENCIO can cause immune-mediated nephritis with renal dysfunction. Withhold BAVENCIO for Grade 2 or Grade 3, and permanently discontinue for Grade 4 increased blood creatinine. Immune-mediated nephritis with renal dysfunction occurred in 0.1% (2/1854) of patients, including Grade 3 (0.1%) and Grade 2 (0.1%) adverse reactions. Systemic corticosteroids were required in all (2/2) patients with nephritis with renal dysfunction.
BAVENCIO can cause immune-mediated dermatologic adverse reactions, including rash or dermatitis. Exfoliative dermatitis including Stevens Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/PD-L1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes. Withhold BAVENCIO for suspected and permanently discontinue for confirmed SJS, TEN, or DRESS. Immune-mediated dermatologic adverse reactions occurred in 6% (108/1854) of patients, including Grade 3 (0.1%) and Grade 2 (1.9%) adverse reactions. Systemic corticosteroids were required in 25% (27/108) of patients with dermatologic adverse reactions.
BAVENCIO can result in other immune-mediated adverse reactions. Other clinically significant immune-mediated adverse reactions occurred at an incidence of <1% in patients who received BAVENCIO or were reported with the use of other PD-1/PD-L1 blocking antibodies. For myocarditis, permanently discontinue BAVENCIO for Grade 2, Grade 3, or Grade 4. For neurological toxicities, withhold BAVENCIO for Grade 2 and permanently discontinue for Grade 3 or Grade 4.
BAVENCIO can cause severe or life-threatening infusion-related reactions. Premedicate patients with an antihistamine and acetaminophen prior to the first 4 infusions and for subsequent infusions based upon clinical judgment and presence/severity of prior infusion reactions. Monitor patients for signs and symptoms of infusion-related reactions, including pyrexia, chills, flushing, hypotension, dyspnea, wheezing, back pain, abdominal pain, and urticaria. Interrupt or slow the rate of infusion for Grade 1 or Grade 2 infusion-related reactions. Permanently discontinue BAVENCIO for Grade 3 or Grade 4 infusion-related reactions. Infusion-related reactions occurred in 26% of patients, including three (0.2%) Grade 4 and ten (0.5%) Grade 3 infusion-related reactions. Eleven (85%) of the 13 patients with Grade ≥3 reactions were treated with intravenous corticosteroids.
Fatal and other serious complications of allogeneic hematopoietic stem cell transplantation (HSCT) can occur in patients who receive HSCT before or after being treated with a PD-1/PD-L1 blocking antibody. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/PD-L1 blocking antibody prior to or after an allogeneic HSCT.
BAVENCIO can cause fetal harm when administered to a pregnant woman. Advise patients of the potential risk to a fetus including the risk of fetal death. Advise females of childbearing potential to use effective contraception during treatment with BAVENCIO and for at least 1 month after the last dose of BAVENCIO. It is not known whether BAVENCIO is excreted in human milk. Advise a lactating woman not to breastfeed during treatment and for at least 1 month after the last dose of BAVENCIO due to the potential for serious adverse reactions in breastfed infants.
The most common adverse reactions (all grades, ≥20%) in patients with metastatic Merkel cell carcinoma (MCC) were fatigue (47%), musculoskeletal pain (29%), infusion-related reaction (26%), rash (25%), nausea (23%), constipation (22%), cough (22%), and diarrhea (21%).
Laboratory abnormalities worsening from baseline (all grades, ≥20%) in patients with metastatic MCC were decreased lymphocyte count (51%), decreased hemoglobin (40%), increased aspartate aminotransferase (31%), decreased platelet count (23%), increased alanine aminotransferase (22%), and increased lipase (21%).
INDICATIONS
BAVENCIO® (avelumab) is indicated for the treatment of adults and pediatric patients 12 years and older with metastatic Merkel cell carcinoma (MCC).
Please see full Prescribing Information and Medication Guide.
COOKIE SETTINGS
©2025 Merck KGaA, Darmstadt, Germany or its affiliates. All rights reserved. EMD Serono is the
Healthcare business of Merck KGaA, Darmstadt, Germany in the U.S. and Canada. BAVENCIO and
CoverOne are registered trademarks of Merck KGaA, Darmstadt, Germany or its affiliates.
US-AVE-01845 04/25
US-AVE-01309 07/24
This site contains medical information that is intended for residents of the United States only and is not meant to substitute for the advice provided by a medical professional. Always consult a physician if you have health concerns. Use and access of this site is subject to the terms and conditions as set out in our Legal Statement and Privacy Policy. This site contains information that is intended for US healthcare professionals only and governed by U.S. laws and government regulations. Canadian residents should consult the EMD Serono Canada Inc. website at www.emdserono.ca for information on products and services approved in Canada.
IMPORTANT SAFETY INFORMATION AND INDICATIONS
IMPORTANT SAFETY INFORMATION
BAVENCIO can cause severe and fatal immune-mediated adverse reactions in any organ system or tissue and at any time after starting treatment with a PD-1/PD-L1 blocking antibody, including after discontinuation of treatment.
Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of PD-1/PD-L1 blocking antibodies. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
No dose reduction for BAVENCIO is recommended. For immune-mediated adverse reactions, withhold or permanently discontinue BAVENCIO depending on severity. In general, withhold BAVENCIO for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue BAVENCIO for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating corticosteroids. In general, if BAVENCIO requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy. Toxicity management guidelines for adverse reactions that do not necessarily require systemic corticosteroids (eg, endocrinopathies and dermatologic reactions) are discussed in subsequent sections.
BAVENCIO can cause immune-mediated pneumonitis. Withhold BAVENCIO for Grade 2, and permanently discontinue for Grade 3 or Grade 4 pneumonitis. Immune-mediated pneumonitis occurred in 1.1% (21/1854) of patients, including fatal (0.1%), Grade 4 (0.1%), Grade 3 (0.3%), and Grade 2 (0.6%) adverse reactions. Systemic corticosteroids were required in all (21/21) patients with pneumonitis.
BAVENCIO can cause immune-mediated colitis. The primary component of immune-mediated colitis consisted of diarrhea. Cytomegalovirus infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies. Withhold BAVENCIO for Grade 2 or Grade 3, and permanently discontinue for Grade 4 colitis. Immune-mediated colitis occurred in 1.5% (27/1854) of patients, including Grade 3 (0.4%) and Grade 2 (0.8%) adverse reactions. Systemic corticosteroids were required in all (27/27) patients with colitis.
BAVENCIO can cause hepatotoxicity and immune-mediated hepatitis. Withhold or permanently discontinue BAVENCIO based on tumor involvement of the liver and severity of aspartate aminotransferase (AST), alanine aminotransferase (ALT), or total bilirubin elevation. Immune-mediated hepatitis occurred with BAVENCIO as a single agent in 1.1% (20/1854) of patients, including fatal (0.1%), Grade 3 (0.8%), and Grade 2 (0.2%) adverse reactions. Systemic corticosteroids were required in all (20/20) patients with hepatitis.
BAVENCIO can cause primary or secondary immune-mediated adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement, as clinically indicated. Withhold BAVENCIO for Grade 3 or Grade 4 endocrinopathies until clinically stable or permanently discontinue depending on severity. Immune-mediated adrenal insufficiency occurred in 0.6% (11/1854) of patients, including Grade 3 (0.1%) and Grade 2 (0.4%) adverse reactions. Systemic corticosteroids were required in all (11/11) patients with adrenal insufficiency.
BAVENCIO can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism. Initiate hormone replacement, as clinically indicated. Withhold BAVENCIO for Grade 3 or Grade 4 endocrinopathies until clinically stable or permanently discontinue depending on severity. Immune-mediated pituitary disorders occurred in 0.1% (1/1854) of patients, which was a Grade 2 (0.1%) adverse reaction.
BAVENCIO can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement for hypothyroidism or institute medical management of hyperthyroidism, as clinically indicated. Withhold BAVENCIO for Grade 3 or Grade 4 endocrinopathies until clinically stable or permanently discontinue depending on severity. Thyroiditis occurred in 0.2% (4/1854) of patients, including Grade 2 (0.1%) adverse reactions. Hyperthyroidism occurred in 0.4% (8/1854) of patients, including Grade 2 (0.3%) adverse reactions. Systemic corticosteroids were required in 25% (2/8) of patients with hyperthyroidism. Hypothyroidism occurred in 5% (97/1854) of patients, including Grade 3 (0.2%) and Grade 2 (3.6%) adverse reactions. Systemic corticosteroids were required in 6% (6/97) of patients with hypothyroidism.
BAVENCIO can cause immune-mediated type I diabetes mellitus, which can present with diabetic ketoacidosis. Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Withhold BAVENCIO for Grade 3 or Grade 4 endocrinopathies until clinically stable or permanently discontinue depending on severity. Immune-mediated type I diabetes mellitus occurred in 0.2% (3/1854) of patients, including Grade 3 (0.2%) adverse reactions.
BAVENCIO can cause immune-mediated nephritis with renal dysfunction. Withhold BAVENCIO for Grade 2 or Grade 3, and permanently discontinue for Grade 4 increased blood creatinine. Immune-mediated nephritis with renal dysfunction occurred in 0.1% (2/1854) of patients, including Grade 3 (0.1%) and Grade 2 (0.1%) adverse reactions. Systemic corticosteroids were required in all (2/2) patients with nephritis with renal dysfunction.
BAVENCIO can cause immune-mediated dermatologic adverse reactions, including rash or dermatitis. Exfoliative dermatitis including Stevens Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/PD-L1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes. Withhold BAVENCIO for suspected and permanently discontinue for confirmed SJS, TEN, or DRESS. Immune-mediated dermatologic adverse reactions occurred in 6% (108/1854) of patients, including Grade 3 (0.1%) and Grade 2 (1.9%) adverse reactions. Systemic corticosteroids were required in 25% (27/108) of patients with dermatologic adverse reactions.
BAVENCIO can result in other immune-mediated adverse reactions. Other clinically significant immune-mediated adverse reactions occurred at an incidence of <1% in patients who received BAVENCIO or were reported with the use of other PD-1/PD-L1 blocking antibodies. For myocarditis, permanently discontinue BAVENCIO for Grade 2, Grade 3, or Grade 4. For neurological toxicities, withhold BAVENCIO for Grade 2 and permanently discontinue for Grade 3 or Grade 4.
BAVENCIO can cause severe or life-threatening infusion-related reactions. Premedicate patients with an antihistamine and acetaminophen prior to the first 4 infusions and for subsequent infusions based upon clinical judgment and presence/severity of prior infusion reactions. Monitor patients for signs and symptoms of infusion-related reactions, including pyrexia, chills, flushing, hypotension, dyspnea, wheezing, back pain, abdominal pain, and urticaria. Interrupt or slow the rate of infusion for Grade 1 or Grade 2 infusion-related reactions. Permanently discontinue BAVENCIO for Grade 3 or Grade 4 infusion-related reactions. Infusion-related reactions occurred in 26% of patients, including three (0.2%) Grade 4 and ten (0.5%) Grade 3 infusion-related reactions. Eleven (85%) of the 13 patients with Grade ≥3 reactions were treated with intravenous corticosteroids.
Fatal and other serious complications of allogeneic hematopoietic stem cell transplantation (HSCT) can occur in patients who receive HSCT before or after being treated with a PD-1/PD-L1 blocking antibody. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/PD-L1 blocking antibody prior to or after an allogeneic HSCT.
BAVENCIO can cause fetal harm when administered to a pregnant woman. Advise patients of the potential risk to a fetus including the risk of fetal death. Advise females of childbearing potential to use effective contraception during treatment with BAVENCIO and for at least 1 month after the last dose of BAVENCIO. It is not known whether BAVENCIO is excreted in human milk. Advise a lactating woman not to breastfeed during treatment and for at least 1 month after the last dose of BAVENCIO due to the potential for serious adverse reactions in breastfed infants.
The most common adverse reactions (all grades, ≥20%) in patients with metastatic Merkel cell carcinoma (MCC) were fatigue (47%), musculoskeletal pain (29%), infusion-related reaction (26%), rash (25%), nausea (23%), constipation (22%), cough (22%), and diarrhea (21%).
Laboratory abnormalities worsening from baseline (all grades, ≥20%) in patients with metastatic MCC were decreased lymphocyte count (51%), decreased hemoglobin (40%), increased aspartate aminotransferase (31%), decreased platelet count (23%), increased alanine aminotransferase (22%), and increased lipase (21%).
INDICATIONS
BAVENCIO® (avelumab) is indicated for the treatment of adults and pediatric patients 12 years and older with metastatic Merkel cell carcinoma (MCC).
Please see full Prescribing Information and Medication Guide.
COOKIE SETTINGS
©2025 Merck KGaA, Darmstadt, Germany or its affiliates. All rights reserved. EMD Serono is the
Healthcare business of Merck KGaA, Darmstadt, Germany in the U.S. and Canada. BAVENCIO and
CoverOne are registered trademarks of Merck KGaA, Darmstadt, Germany or its affiliates.
US-AVE-01845 04/25
US-AVE-01845 04/25
This site contains medical information that is intended for residents of the United States only and is not meant to substitute for the advice provided by a medical professional. Always consult a physician if you have health concerns. Use and access of this site is subject to the terms and conditions as set out in our Legal Statement and Privacy Policy. This site contains information that is intended for US healthcare professionals only and governed by U.S. laws and government regulations. Canadian residents should consult the EMD Serono Canada Inc. website at www.emdserono.ca for information on products and services approved in Canada.

The content on this website is intended for US healthcare professionals. By entering this website, you confirm you are a US healthcare professional.


