BAVENCIO is not a cure. The data represent an average of patients in the clinical trial, and not all patients will experience the same results.
See how BAVENCIO® (avelumab) was studied as a maintenance treatment in certain patients with advanced urothelial carcinoma (UC)
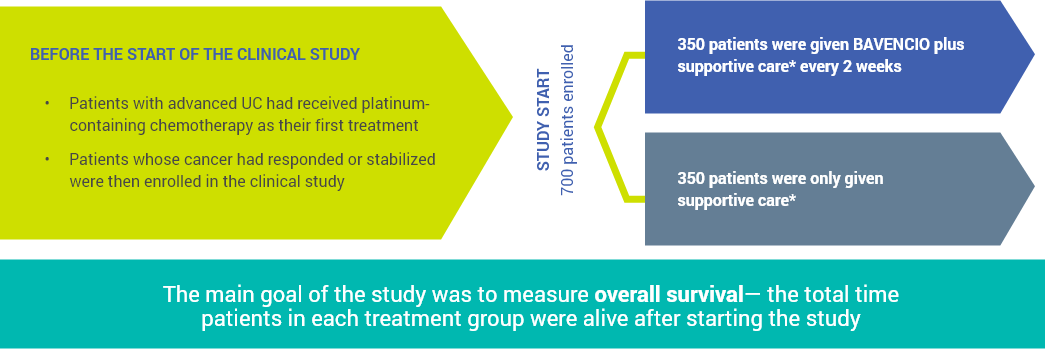
*Supportive care included antibiotics, nutritional support, hydration, and pain management.
†In some cancer types, when cancer cells have high amounts of a protein called PD-L1, they can trick the immune system and avoid being attacked as harmful substances. Speak to your doctor to learn more about the role of PD-L1 in advanced UC.
PD-L1=programmed death ligand 1.
Common side effects seen in the clinical study
The most common side effects of BAVENCIO as maintenance treatment in people with urothelial carcinoma (UC) whose cancer responded or stabilized after platinum-containing chemotherapy as first treatment include: feeling tired, muscle and bone pain, urinary tract infection, and rash.
Click here to see full Important Safety Information
BAVENCIO plus supportive care helped some patients live longer than those on supportive care alone
CLINICAL STUDY RESULTS
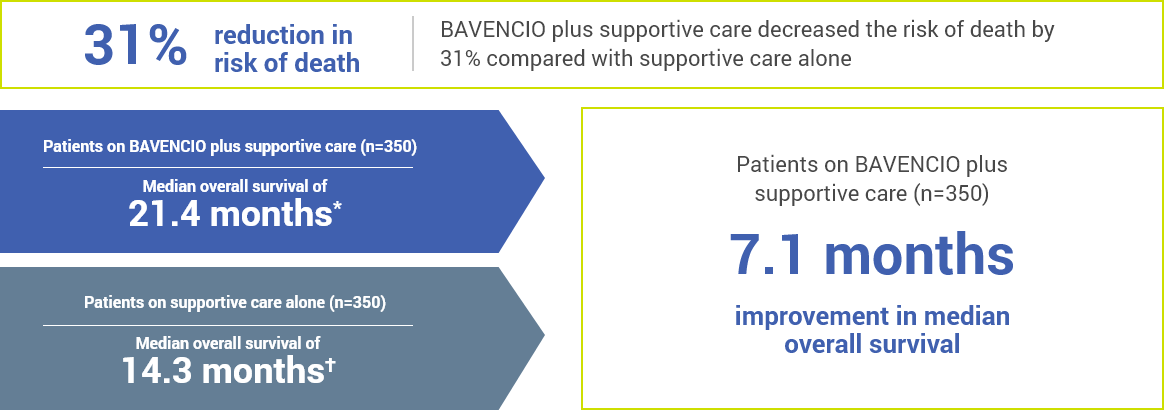
*Half the patients lived longer than 21.4 months and the other half lived less than 21.4 months.
†Half the patients lived longer than 14.3 months and the other half lived less than 14.3 months.
FOLLOW-UP ANALYSIS
In a follow-up analysis that was completed on June 4, 2021:


*Half the patients lived longer than 23.8 months, and the other half lived less than 23.8 months.
†Half the patients lived longer than 15.0 months, and the other half lived less than 15.0 months.
BAVENCIO can be used as a maintenance treatment, regardless of PD-L1 status.
INDICATIONS & IMPORTANT SAFETY INFORMATION
Important Safety Information
Important Safety Information
BAVENCIO® (avelumab) is a medicine that may treat certain cancers by working with your immune system. BAVENCIO can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life-threatening and can lead to death. You can have more than one of these problems at the same time. These problems may happen anytime during treatment or even after your treatment has ended.
Getting medical treatment right away may help keep these problems from becoming more serious. Your healthcare provider will check you for these problems during your treatment with BAVENCIO. Your healthcare provider may treat you with corticosteroids or hormone replacement medicines. Your healthcare provider may also need to delay or completely stop treatment with BAVENCIO if you have severe side effects.
BAVENCIO can cause serious side effects. Call or see your healthcare provider right away if you get any new or worsening signs or symptoms, including:
Lung problems
- cough
- shortness of breath
- chest pain
Intestinal problems
- diarrhea (loose stools) or more frequent bowel movements than usual
- stools that are black, tarry, sticky, or have blood or mucus
- severe stomach-area (abdomen) pain or tenderness
Liver problems
- yellowing of your skin or the whites of your eyes
- severe nausea or vomiting
- pain on the right side of your stomach area (abdomen)
- dark urine (tea colored)
- bleeding or bruising more easily than normal
Hormone gland problems
- headache that will not go away or unusual headaches
- eye sensitivity to light
- eye problems
- rapid heartbeat
- increased sweating
- extreme tiredness
- weight gain or weight loss
- feeling more hungry or thirsty than usual
- urinating more often than usual
- hair loss
- feeling cold
- constipation
- your voice gets deeper
- dizziness or fainting
- changes in mood or behavior, such as decreased sex drive, irritability, or forgetfulness
Kidney problems
- decrease in your amount of urine
- blood in your urine
- swelling of your ankles
- loss of appetite
Skin problems
- rash
- itching
- skin blistering or peeling
- painful sores or ulcers in mouth or nose, throat, or genital area
- fever or flu-like symptoms
- swollen lymph nodes
Problems can also happen in other organs and tissues. These are not all of the signs or symptoms of immune system problems that can happen with BAVENCIO. Call or see your healthcare provider right away for any new or worsening signs or symptoms, which may include:
- Chest pain, irregular heartbeat, shortness of breath or swelling of ankles
- Confusion, sleepiness, memory problems, changes in mood or behavior, stiff neck, balance problems, tingling or numbness of the arms or legs
- Double vision, blurry vision, sensitivity to light, eye pain, changes in eye sight
- Persistent or severe muscle pain or weakness, muscle cramps
- Low red blood cells, bruising
Infusion-related reactions can sometimes be severe or life-threatening. Signs and symptoms of infusion-related reactions may include:
- chills or shaking
- hives
- flushing
- shortness of breath or wheezing
- dizziness
- feel like passing out
- fever
- back pain
- stomach-area (abdomen) pain
Complications, including graft-versus-host-disease (GVHD), in people who have received a bone marrow (stem cell) transplant that uses donor stem cells (allogeneic). These complications can be serious and can lead to death. These complications may happen if you underwent transplantation either before or after being treated with BAVENCIO. Your healthcare provider will monitor you for these complications.
Before you receive BAVENCIO, tell your healthcare provider about all of your medical conditions, including if you:
- have immune system problems such as Crohn’s disease, ulcerative colitis, or lupus
- have received an organ transplant
- have received or plan to receive a stem cell transplant that uses donor stem cells (allogeneic)
- have a condition that affects your nervous system, such as myasthenia gravis or Guillain-Barré syndrome
- have heart problems or high blood pressure
- have diabetes
- have a high cholesterol level in your blood
- are pregnant or plan to become pregnant. BAVENCIO can harm your unborn baby
- You should use an effective method of birth control during your treatment and for at least 1 month after the last dose of BAVENCIO. Talk to your healthcare provider about birth control methods that you can use during this time
Females who are able to become pregnant:
- are breastfeeding or plan to breastfeed. It is not known if BAVENCIO passes into your breast milk. Do not breastfeed during treatment and for at least 1 month after the last dose of BAVENCIO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
The most common side effects of BAVENCIO as maintenance treatment in people with urothelial carcinoma (UC) whose cancer responded or stabilized after platinum-containing chemotherapy as first treatment include:
- feeling tired
- muscle and bone pain
- urinary tract infection
- rash
The most common side effects of BAVENCIO in people with UC after platinum-containing chemotherapy that did not work, or is no longer working, include:
- feeling tired
- infusion-related reactions including chills, fever, back pain, redness, and shortness of breath
- muscle and bone pain
- nausea
- decreased appetite
- urinary tract infection
These are not all the possible side effects of BAVENCIO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including Medication Guide for patients.
Indications
BAVENCIO is a prescription medicine used to treat:
- a type of cancer in the bladder or urinary tract called urothelial carcinoma (UC) when it has spread or cannot be removed by surgery (advanced UC). BAVENCIO may be used:
- as maintenance treatment when your cancer has responded or stabilized after you have received platinum-containing chemotherapy as your first treatment
- when you have received platinum-containing chemotherapy, and it did not work or is no longer working
It is not known if BAVENCIO is safe and effective in children under the age of 12.
These are not all the possible side effects of BAVENCIO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including Medication Guide for patients.
COOKIE SETTINGS
©2025 Merck KGaA, Darmstadt, Germany or its affiliates. All rights reserved.
EMD Serono is the Healthcare business of Merck KGaA, Darmstadt, Germany in the U.S. and Canada.
BAVENCIO, the Bavencio Connect Mentor Program, and CoverOne are registered trademarks of Merck KGaA,
Darmstadt, Germany or its affiliates.
US-AVE-01894 09/25
US-AVE-01894 09/25
This site contains medical information that is intended for residents of the United States only and is not meant to substitute for the advice provided by a medical professional. Always consult a physician if you have health concerns. Use and access of this site is subject to the terms and conditions as set out in our Legal Statement and Privacy Policy. This site contains information that is intended for US residents only. Canadian residents should consult the EMD Serono Canada Inc. website at www.emdserono.ca for information on products and services approved in Canada.
Important Safety Information
BAVENCIO® (avelumab) is a medicine that may treat certain cancers by working with your immune system. BAVENCIO can cause your immune system to attack normal organs and tissues in any area of your body and can affect the way they work. These problems can sometimes become severe or life-threatening and can lead to death. You can have more than one of these problems at the same time. These problems may happen anytime during treatment or even after your treatment has ended.
Getting medical treatment right away may help keep these problems from becoming more serious. Your healthcare provider will check you for these problems during your treatment with BAVENCIO. Your healthcare provider may treat you with corticosteroids or hormone replacement medicines. Your healthcare provider may also need to delay or completely stop treatment with BAVENCIO if you have severe side effects.
BAVENCIO can cause serious side effects. Call or see your healthcare provider right away if you get any new or worsening signs or symptoms, including:
Lung problems
- cough
- shortness of breath
- chest pain
Intestinal problems
- diarrhea (loose stools) or more frequent bowel movements than usual
- stools that are black, tarry, sticky, or have blood or mucus
- severe stomach-area (abdomen) pain or tenderness
Liver problems
- yellowing of your skin or the whites of your eyes
- severe nausea or vomiting
- pain on the right side of your stomach area (abdomen)
- dark urine (tea colored)
- bleeding or bruising more easily than normal
Hormone gland problems
- headache that will not go away or unusual headaches
- eye sensitivity to light
- eye problems
- rapid heartbeat
- increased sweating
- extreme tiredness
- weight gain or weight loss
- feeling more hungry or thirsty than usual
- urinating more often than usual
- hair loss
- feeling cold
- constipation
- your voice gets deeper
- dizziness or fainting
- changes in mood or behavior, such as decreased sex drive, irritability, or forgetfulness
Kidney problems
- decrease in your amount of urine
- blood in your urine
- swelling of your ankles
- loss of appetite
Skin problems
- rash
- itching
- skin blistering or peeling
- painful sores or ulcers in mouth or nose, throat, or genital area
- fever or flu-like symptoms
- swollen lymph nodes
Problems can also happen in other organs and tissues. These are not all of the signs or symptoms of immune system problems that can happen with BAVENCIO. Call or see your healthcare provider right away for any new or worsening signs or symptoms, which may include:
- Chest pain, irregular heartbeat, shortness of breath or swelling of ankles
- Confusion, sleepiness, memory problems, changes in mood or behavior, stiff neck, balance problems, tingling or numbness of the arms or legs
- Double vision, blurry vision, sensitivity to light, eye pain, changes in eye sight
- Persistent or severe muscle pain or weakness, muscle cramps
- Low red blood cells, bruising
Infusion-related reactions can sometimes be severe or life-threatening.
Signs and symptoms of infusion-related reactions may include:
- chills or shaking
- hives
- flushing
- shortness of breath or wheezing
- dizziness
- feel like passing out
- fever
- back pain
- stomach-area (abdomen) pain
Complications, including graft-versus-host-disease (GVHD), in people who have received a bone marrow (stem cell) transplant that uses donor stem cells (allogeneic).
These complications can be serious and can lead to death. These complications may happen if you underwent transplantation either before or after being treated with BAVENCIO. Your healthcare provider will monitor you for these complications.
Before you receive BAVENCIO, tell your healthcare provider about all of your medical conditions, including if you:
- have immune system problems such as Crohn’s disease, ulcerative colitis, or lupus
- have received an organ transplant
- have received or plan to receive a stem cell transplant that uses donor stem cells (allogeneic)
- have a condition that affects your nervous system, such as myasthenia gravis or Guillain-Barré syndrome
- have heart problems or high blood pressure
- have diabetes
- have a high cholesterol level in your blood
- are pregnant or plan to become pregnant. BAVENCIO can harm your unborn baby
- You should use an effective method of birth control during your treatment and for at least 1 month after the last dose of BAVENCIO. Talk to your healthcare provider about birth control methods that you can use during this time
Females who are able to become pregnant:
- are breastfeeding or plan to breastfeed. It is not known if BAVENCIO passes into your breast milk. Do not breastfeed during treatment and for at least 1 month after the last dose of BAVENCIO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
The most common side effects of BAVENCIO as maintenance treatment in people with urothelial carcinoma (UC) whose cancer responded or stabilized after platinum-containing chemotherapy as first treatment include:
- feeling tired
- muscle and bone pain
- urinary tract infection
- rash
The most common side effects of BAVENCIO in people with UC after platinum-containing chemotherapy that did not work, or is no longer working, include:
- feeling tired
- infusion-related reactions including chills, fever, back pain, redness, and shortness of breath
- muscle and bone pain
- nausea
- decreased appetite
- urinary tract infection
These are not all the possible side effects of BAVENCIO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including Medication Guide for patients.
Indications
BAVENCIO is a prescription medicine used to treat:
- a type of cancer in the bladder or urinary tract called urothelial carcinoma (UC) when it has spread or cannot be removed by surgery (advanced UC). BAVENCIO may be used:
- as maintenance treatment when your cancer has responded or stabilized after you have received platinum-containing chemotherapy as your first treatment
- when you have received platinum-containing chemotherapy, and it did not work or is no longer working
It is not known if BAVENCIO is safe and effective in children under the age of 12.
These are not all the possible side effects of BAVENCIO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including Medication Guide for patients.
COOKIE SETTINGS
©2025 Merck KGaA, Darmstadt, Germany or its affiliates. All rights reserved.
EMD Serono is the Healthcare business of Merck KGaA, Darmstadt, Germany in the U.S. and Canada.
BAVENCIO, the Bavencio Connect Mentor Program, and CoverOne are registered trademarks of Merck KGaA, Darmstadt, Germany or its affiliates.
US-AVE-01894 09/25
US-AVE-01894 09/25
This site contains medical information that is intended for residents of the United States only and is not meant to substitute for the advice provided by a medical professional. Always consult a physician if you have health concerns. Use and access of this site is subject to the terms and conditions as set out in our Legal Statement and Privacy Policy. This site contains information that is intended for US residents only. Canadian residents should consult the EMD Serono Canada Inc. website at www.emdserono.ca for information on products and services approved in Canada.


