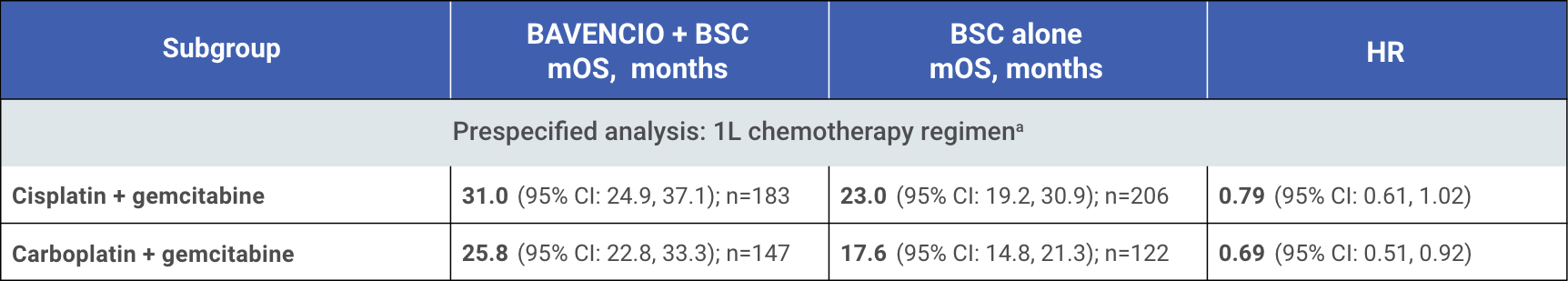- Primary and Long-Term Analyses
- Long-Term Exploratory Analyses
Extend Overall Survival With BAVENCIO1,2
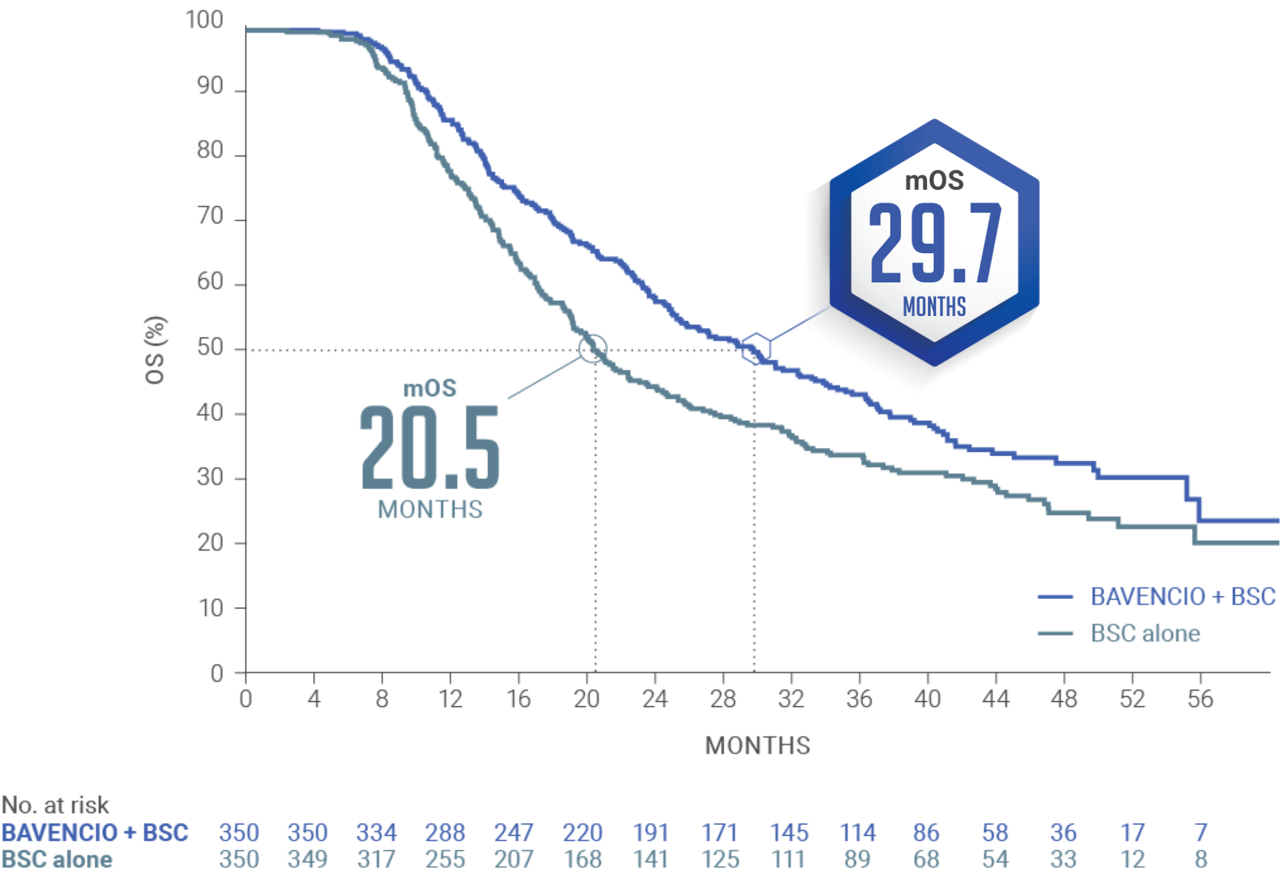
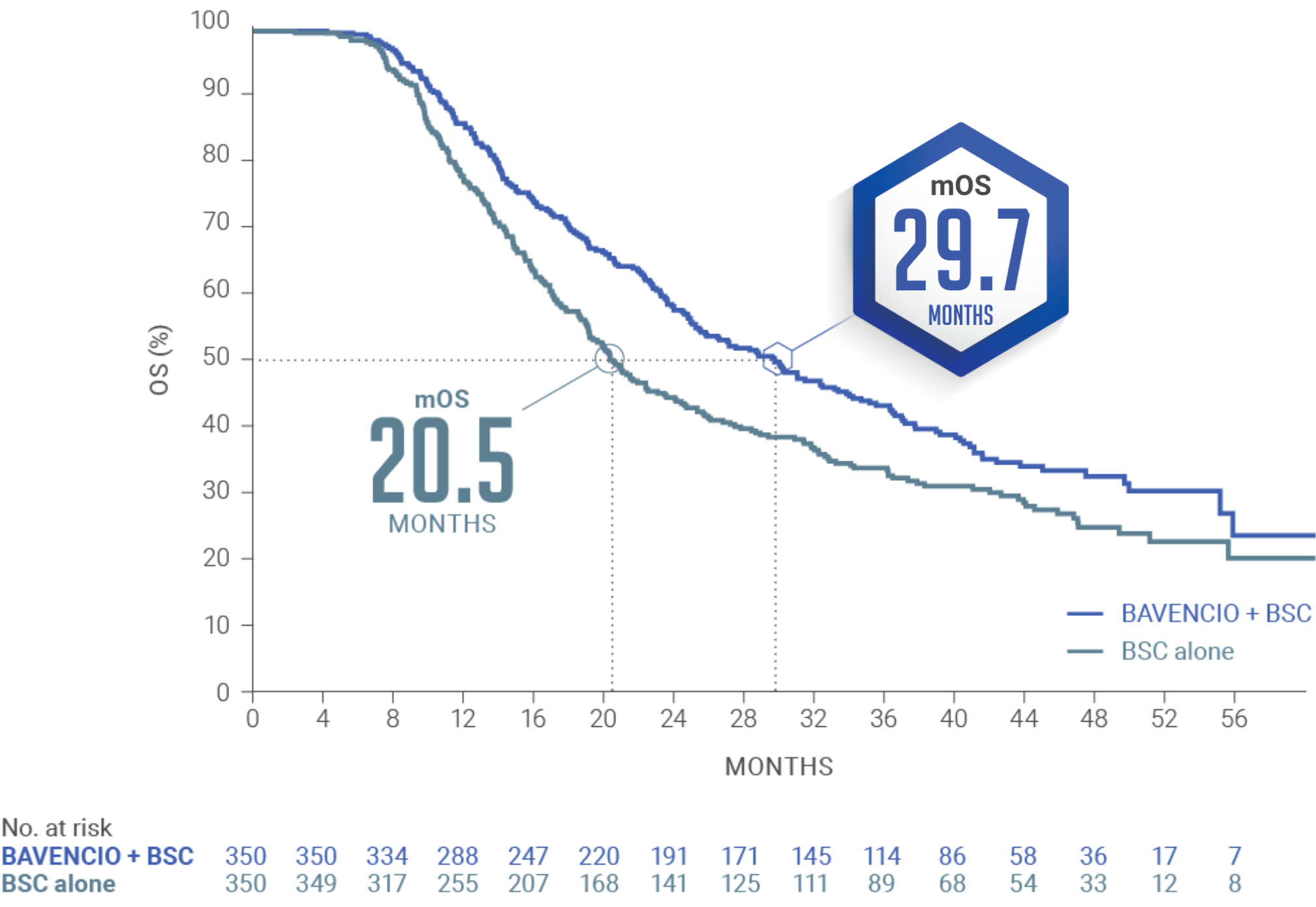
JAVELIN BLADDER 100 PRIMARY ANALYSIS: BAVENCIO + BSC demonstrated superior OS vs BSC alone1
Median follow-up: 19.6 months (95% CI: 18.0, 20.6) in the BAVENCIO + BSC arm; 19.2 months (95% CI: 17.4, 21.6) in the BSC-alone arm3
mOS of 21.4 MONTHS (95% CI: 18.9, 26.1) with BAVENCIO + BSC vs 14.3 MONTHS (95% CI: 12.9, 17.9) with BSC alone (n=350 in each arm); HR 0.69 (0.56, 0.86); 2-sided P-valuea=0.001
The pre-planned interim analysis was considered the primary analysis since the primary endpoint was met.2,4
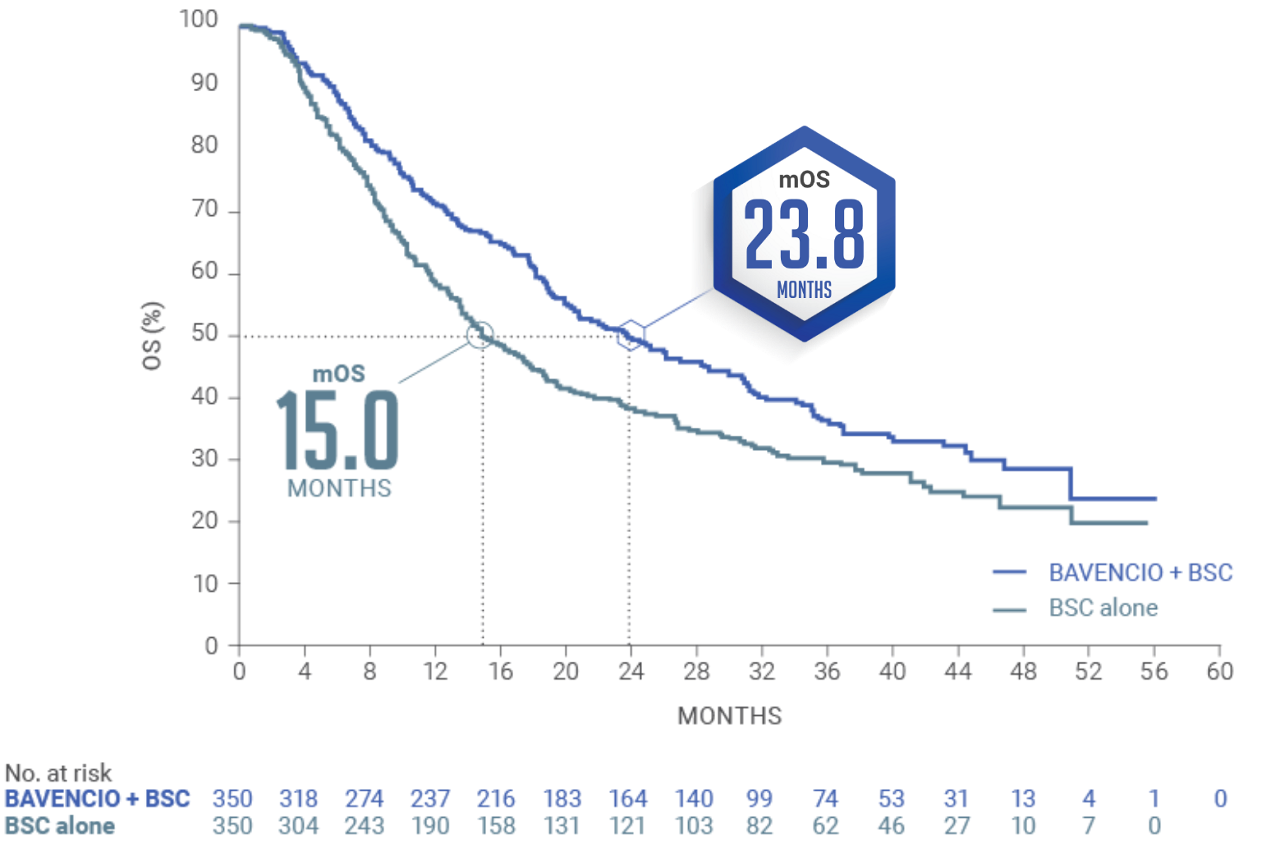
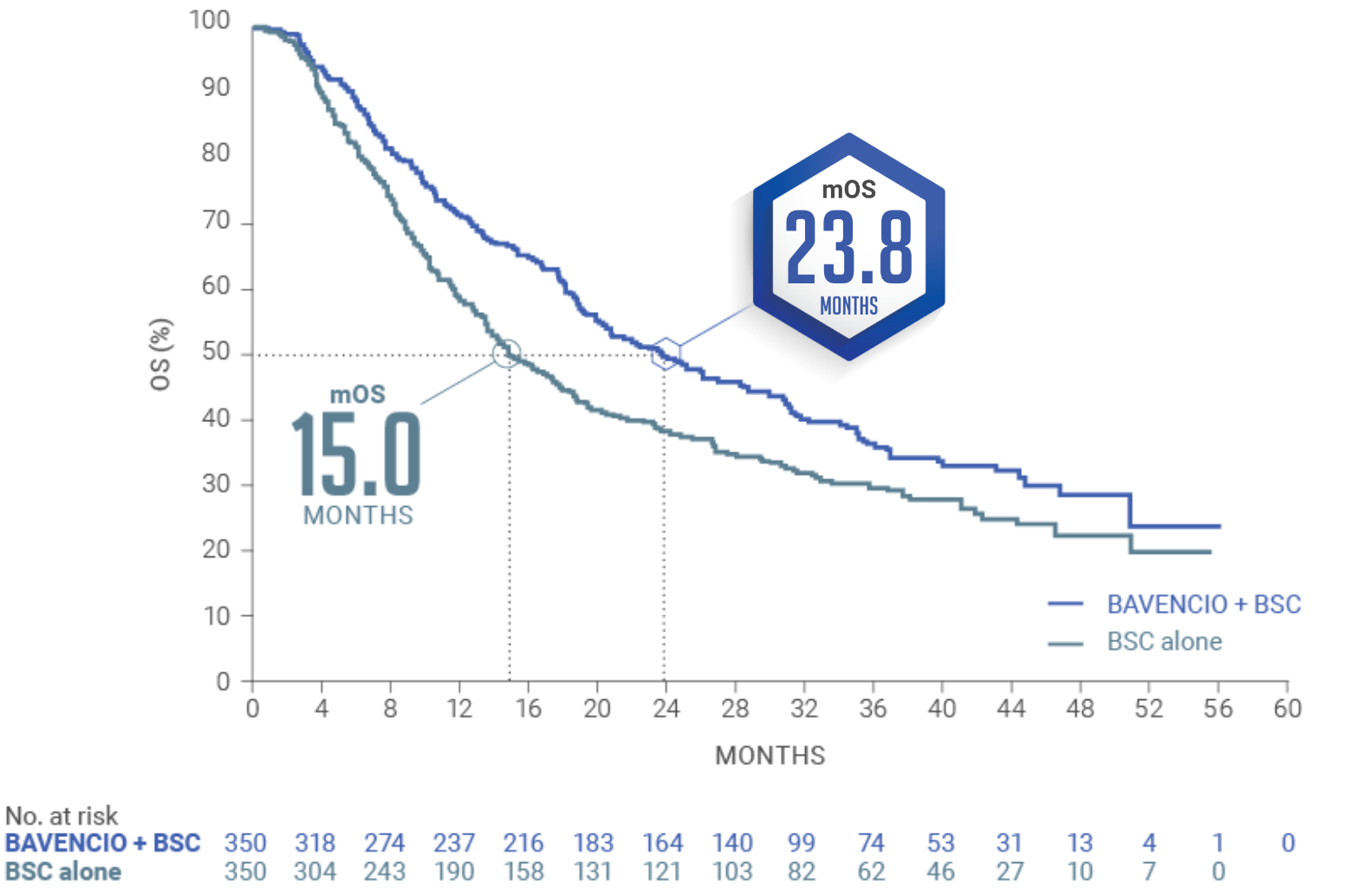
LONG-TERM ANALYSIS (3+ years): Consistent OS results were observed1
Median follow-up: 38.0 months (95% CI: 36.1, 40.5) in the BAVENCIO + BSC arm; 39.6 months (95% CI: 36.2, 41.7) in the BSC-alone arm2,4
Long-term OS results in PD-L1–positive patients1,b (n=358, 51% of patients): HR 0.69 (95% CI: 0.52, 0.90)
PD-L1–negative tumors (exploratory analysis; n=270, 39% of patients): OS HR 0.82 (95% CI: 0.62, 1.09)
An updated OS analysis was conducted when 452 deaths were observed. The follow-up OS analysis was prespecified, but no formal hypothesis testing was performed given that the OS endpoint was met in the initial interim analysis.1,2
aP-value based on stratified log-rank.5
bUsing the VENTANA PD-L1 (SP263) assay, PD-L1–positive status was defined as PD-L1 expression in ≥25% of tumor cells or in ≥25% or 100% of tumor-associated immune cells if the percentage of immune cells was >1% or ≤1%, respectively. If none of these criteria were met, PD-L1 status was considered negative.5
SELECTED SAFETY INFORMATION
BAVENCIO can cause severe and fatal immune-mediated adverse reactions in any organ system or tissue and at any time after starting treatment with a PD-1/PD-L1 blocking antibody, including after discontinuation of treatment.
Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of PD-1/PD-L1 blocking antibodies. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Additional Data from the JAVELIN Bladder 100 Trial
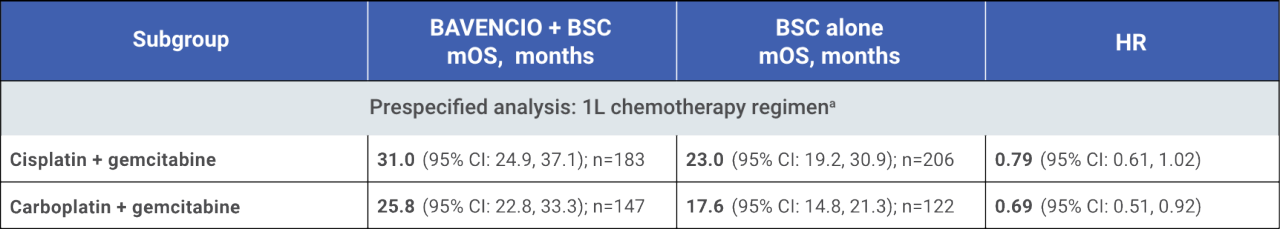
mOS of 〜30 months from start of 1L platinum-containing chemotherapy4
LIMITATIONS:
These are exploratory, post hoc analysis of OS data, inclusive of platinum-containing chemotherapy (4-6 cycles), treatment-free interval (4-10 weeks, per trial protocol),
randomized study treatment with BAVENCIO + BSC or BSC alone, and subsequent therapy. This analysis only includes patients who did not progress on first-line platinum-
containing chemotherapy and subsequently enrolled in the JAVELIN Bladder 100 trial. Small patient numbers can be a limitation of subgroup analyses. Safety data are not
available pre-randomization. No conclusions can be drawn from these OS analyses.
mOS from start of 1L chemotherapy by 1L chemotherapy regimen4
SELECTED SAFETY INFORMATION
No dose reduction for BAVENCIO is recommended. For immune-mediated adverse reactions, withhold or permanently discontinue BAVENCIO depending on severity. In general, withhold BAVENCIO for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue BAVENCIO for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating corticosteroids. In general, if BAVENCIO requires interruption or discontinuation, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy. Toxicity management guidelines for adverse reactions that do not necessarily require systemic corticosteroids (eg, endocrinopathies and dermatologic reactions) are discussed in subsequent sections.
LIMITATIONS6:
- Open-label trial design and the limited number of patients providing data at later time points
- The limited number of patients at later cycles was prominent in the control arm, mainly due to progression events, which may limit the interpretation of longer-term PRO
- The FBlSI-18 instrument was validated in patients with bladder cancer, but some items may be less relevant for advanced disease in the maintenance setting
- All analyses were not adjusted for multiple testing, hindering their overall interpretation
- The methodology of this assessment does not allow conclusions based on this data
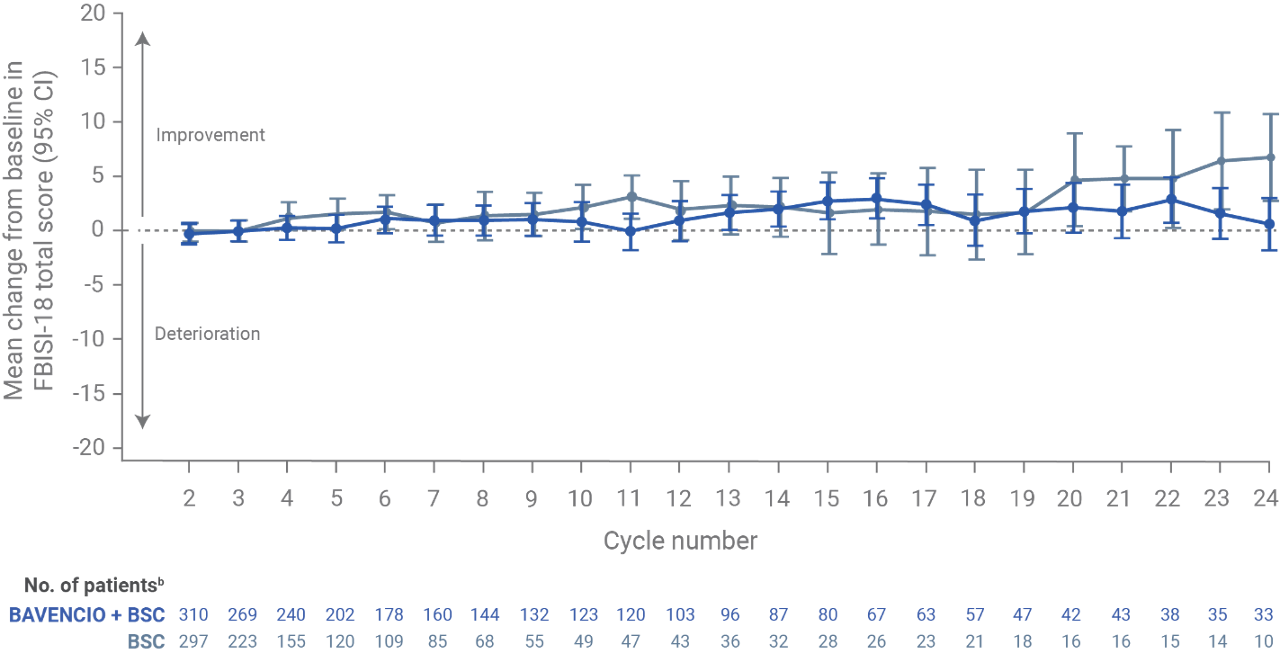
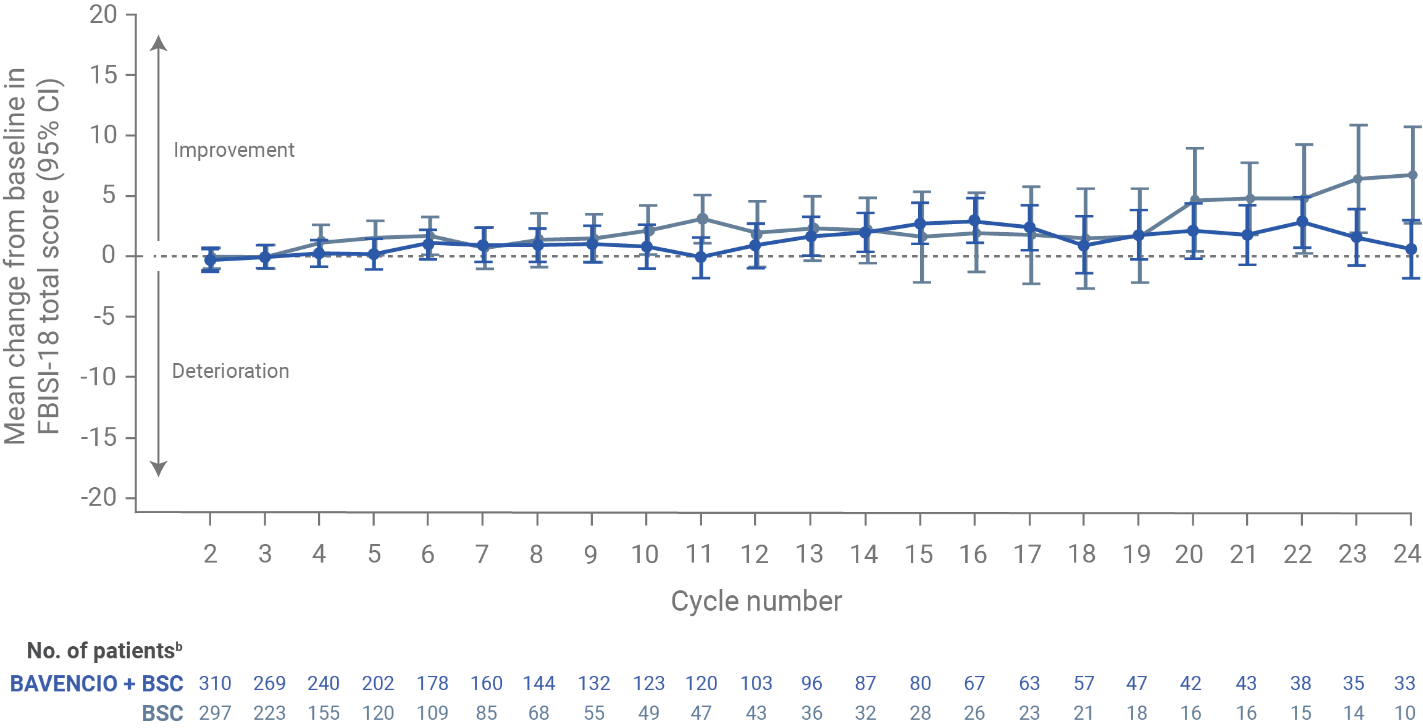
Patient-reported outcomes from JAVELIN Bladder 100: a prespecified secondary endpoint (N=700)6
- NCCN/FBISI-18 is a validated tool that measures symptoms and QoL in the past 7 days in bladder cancer patients
- Among patients in the BAVENCIO + BSC and BSC-alone arms (n=350 for each), overall and average per-assessment completion rates for PRO analyses among eligible patients at each scheduled visit were >90% for most of the treatment period
FBISI-18 analysis in the overall populationa
- At baseline (after completion of chemotherapy but before the start of maintenance treatment) in the overall population, FBISI-18 total scores were similar between the BAVENCIO + BSC arm (mean, 53.3; SD, 9.6) and the BSC-alone arm (mean, 52.7; SD, 9.3)
- The mean change from baseline on treatment appeared relatively stable, with most values at or above baseline and most corresponding CIs including the baseline value
Figure recreated from Grivas P, et al. Eur Urol. 2023;83(4):320-328.
aFBISI-18 subscales focusing on disease-related symptoms: physical (DRS-P; pain, weight loss, urination, weakness, dizziness, meeting family needs, appetite, erection in males, and sleep), emotional (DRS-E; worrying about disease worsening and sadness), treatment side effects (TSEs; nausea, lack of energy, feeling ill, bowel control, and bother of TSE), and functional well-being (FWB; ability to enjoy life and contentment with QOL). Ranges for each FBlSI-18 score: total, 0-72; DRS-P, 0-36; DRS-E, 0-8; TSE, 0-20; FWB, 0-8. Descriptive statistics were calculated for FBlSI-18 total score and subscales. Estimates of clinically important differences and changes for group comparisons: total, 3-6; DRS-P, 2-3; TSE, 1-2; and DRS-E and FWB, one each. Estimates of significant changes in individual patients: total, 3-9; DRS-P, 2-6; DRS-E, 1-3; TSE, 2-5; and FWB, 2-4.
bNumber of patients who completed the baseline assessment and the assessment at the respective cycle. Data for on-treatment visits that had 10 or more patients in both arms are shown. For the BAVENCIO + BSC and BSC-alone arms, 333 and 330 patients responded to one or more items at baseline, respectively.
1L=first line; BSC=best supportive care; CI=confidence interval; FBlSI-18=Functional Assessment of Cancer Therapy Bladder Symptom Index-18; HR=hazard ratio; mOS=median overall survival; OS=overall survival; PD-L1=programmed death ligand-1; PRO=patient-reported outcome; QOL=quality of life; SD=standard deviation.
References: 1. Bavencio Prescribing Information. EMD Serono, Inc.; 2024. 2. Powles T, Park SH, Caserta C, et al. Avelumab first-line maintenance for advanced urothelial carcinoma: results from the JAVELIN Bladder 100 Trial after ≥2 years of follow-up. J Clin Oncol. 2023;41(19):3486-3492. 3. Grivas P, Park SH, Voog E, et al. Avelumab first-line maintenance therapy for advanced urothelial carcinoma: comprehensive clinical subgroup analyses from the JAVELIN Bladder 100 Phase 3 Trial. Eur Urol. 2023;84(1):95-108. 4. Data on file. EMD Serono, Inc., Rockland, MA. 5. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218-1230. 6. Grivas P, Kopyltsov E, Su P-J, et al. Patient-reported outcomes from JAVELIN Bladder 100: avelumab first-line maintenance plus best supportive care versus best supportive care alone for advanced urothelial carcinoma. Eur Urol. 2023;83(4):320-328. 7. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Bladder Cancer V.1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed March 27, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.