PRIMARY ANALYSIS: Adverse Reactions1
- A fatal adverse reaction (sepsis) occurred in one patient (0.3%) receiving BAVENCIO + best supportive care (BSC)
- Serious adverse reactions occurred in 28% of patients receiving BAVENCIO + BSC. Serious adverse reactions in ≥1% of patients included urinary tract infection (including kidney infection, pyelonephritis, and urosepsis) (6.1%), pain (including abdominal, back, bone, flank, extremity, and pelvic pain) (3.2%), acute kidney injury (1.7%), hematuria (1.5%), sepsis (1.2%), and infusion-related reaction (1.2%)
- Patients received premedication with an antihistamine and acetaminophen prior to each infusion. Infusion-related reactions occurred in 10% of patients treated with BAVENCIO + BSC (Grade 3: 0.9%)
- Thirty-one (9%) patients treated with BAVENCIO + BSC received an oral prednisone dose equivalent to ≥40 mg daily for an immune-mediated adverse reaction
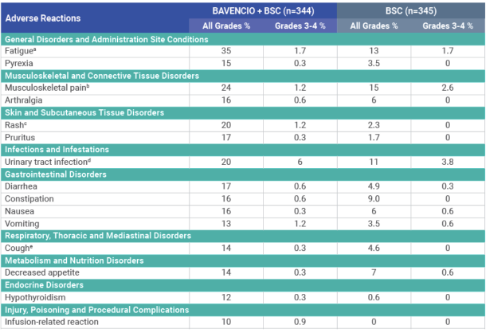
Adverse reactions (≥10%) of patients receiving BAVENCIO + BSC1
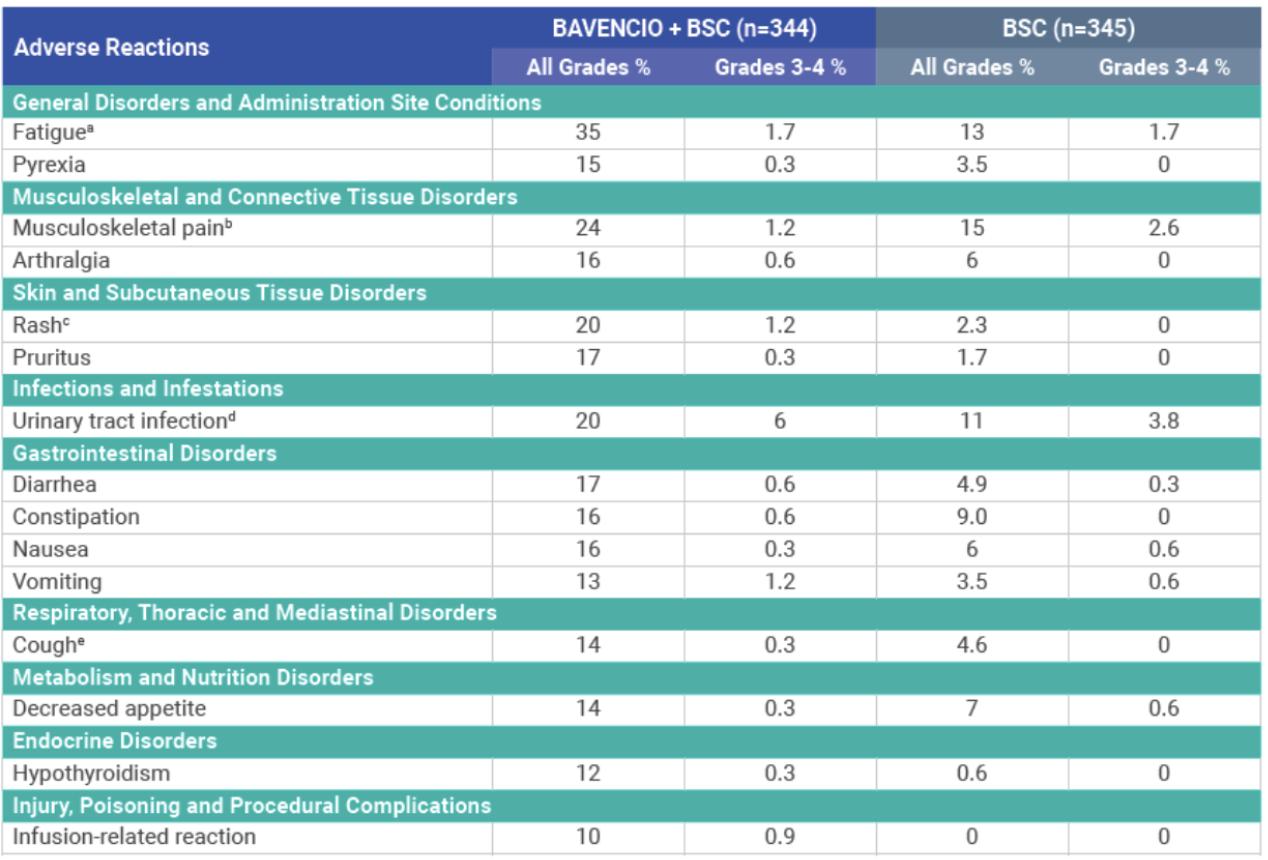
aFatigue is a composite term that includes fatigue, asthenia, and malaise.
bMusculoskeletal pain is a composite term that includes musculoskeletal pain, back pain, myalgia, and neck pain.
cRash is a composite term that includes rash, rash maculopapular, erythema, dermatitis acneiform, eczema, erythema multiforme, rash erythematous, rash macular, rash papular, rash pruritic, drug eruption, and lichen planus.
dUrinary tract infection is a composite term that includes urinary tract infection, urosepsis, cystitis, kidney infection, pyuria, pyelonephritis, bacteriuria, pyelonephritis acute, urinary tract infection bacterial, and Escherichia urinary tract infection.
eCough is a composite term that includes cough and productive cough.
PRIMARY
ANALYSIS3
TRARs (77.3% of patients)
16.6
%
of patients had
a Grade ≥3 TRAR
irARs (29.4% of patients)
7.0
%
of patients had
a Grade ≥3 irAR
Treatment discontinuation due to ARs: 11.9%
Treatment discontinuation due to TRARs: 9.6%
LONG-TERM
ANALYSIS2
TRARs (78.2% of patients)
19.5
%
of patients had
a Grade ≥3 TRAR
irARs (32.3% of patients)
7.6
%
of patients had
a Grade ≥3 irAR
Treatment discontinuation due to ARs: 14.2%
Treatment discontinuation due to TRARs: 11.6%
≥38 MONTHS)2
NEARLY
20%
of patients were still receiving treatment with BAVENCIO
after 2 years (median duration,
5.8 months)2
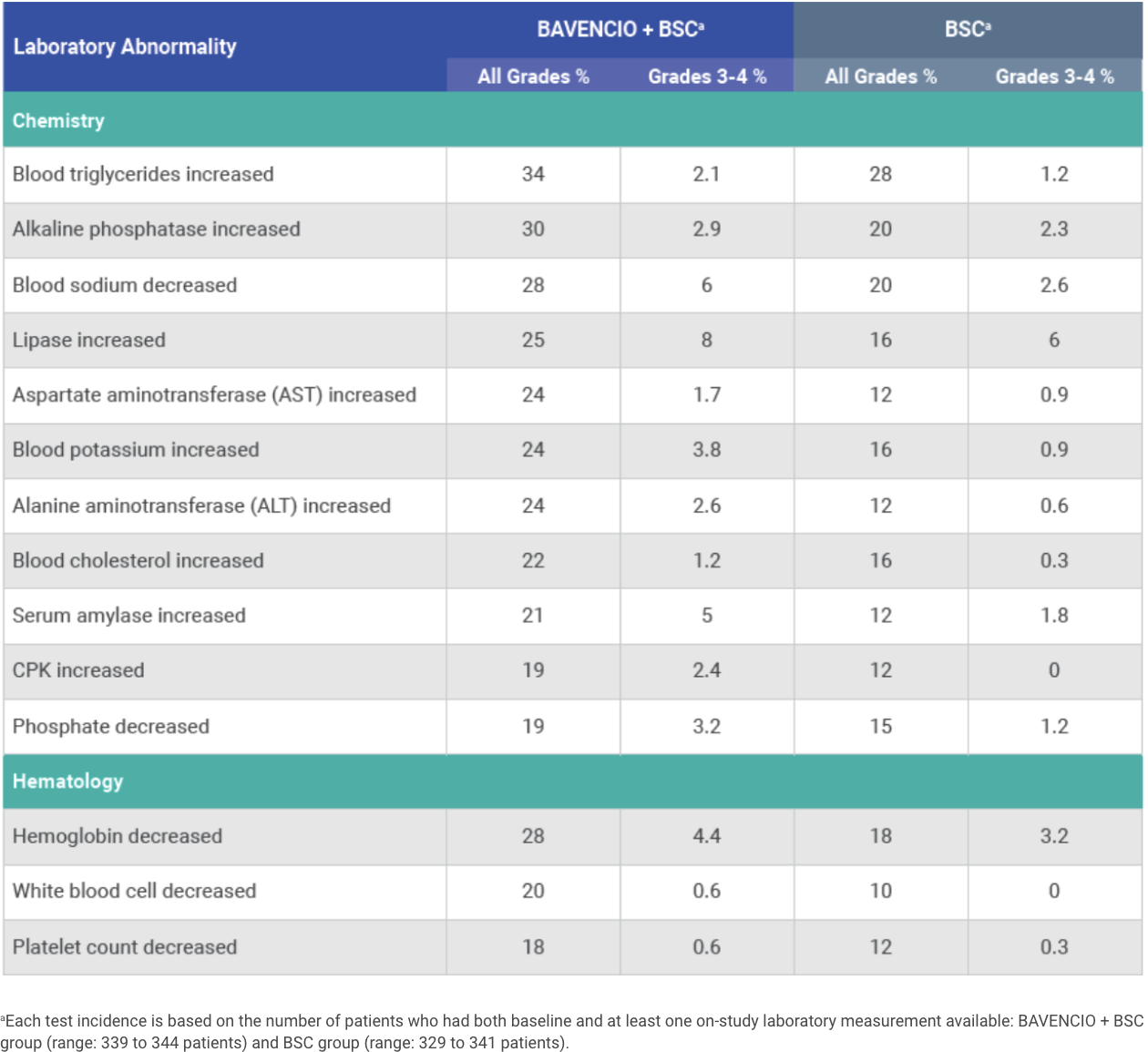
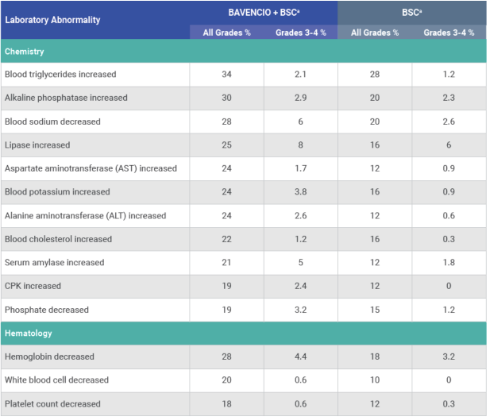
PRIMARY ANALYSIS: Selected laboratory abnormalities worsening from baseline occurring in ≥10% of patients receiving BAVENCIO + BSC1
AR = Adverse reaction; BSC=best supportive care; irAR=immune-mediated adverse reaction; TRAR=treatment-related adverse reaction.
References: 1. Bavencio Prescribing Information. EMD Serono, Inc.; 2024. 2. Powles T, Park SH, Caserta C, et al. Avelumab first-line maintenance for advanced urothelial carcinoma: results from the JAVELIN Bladder 100 Trial after ≥2 years of follow-up. J Clin Oncol. 2023;41(19):3486-3492. 3. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218-1230.